Alzheimer's Disease/Dementia: Oxidative Stress, Vitamin D and Metal Ion Levels
Hello, I am Julie Donaldson and I am a clinical nutritionist with functional medicine training. I specialize in restoring balance in complex, chronic and acute health conditions. I welcome you to peruse other articles that may be of interest to you in your health investigation!
“The onset of dementia with my mom was one of the hardest things my family has ever faced. Julie’s expertise and guidance with multiple phases of support have been invaluable in slowing the progression and giving us all a better quality of life. She is incredibly attentive and intelligent about all the details of these problems. We love her!”
Alzheimer’s Disease (AD) and dementia are in a category of staggering growth in the number of deaths related to these conditions. In a period of 5 years, the death rate in the U.S. increased 17%, while rates doubled from 2000-2019. More than 5 million Americans are living with Alzheimer’s and by 2050, it is estimated there will be as many as 16 million Americans living with the disease. Because reliable testing methods have yet to be identified for early risk measures, those afflicted with Alzheimer's are left to manage symptoms after the disease has taken hold, versus mitigating potential risks of onset and progression. Preventing the diseases may be one of the most critical holistic health needs of our time. In this article, we will explore brand new research on the connections between vitamin D deficiency and dementia. We will also explore the role of ionic metals (such as zinc, copper and iron) in neurodegeneration.
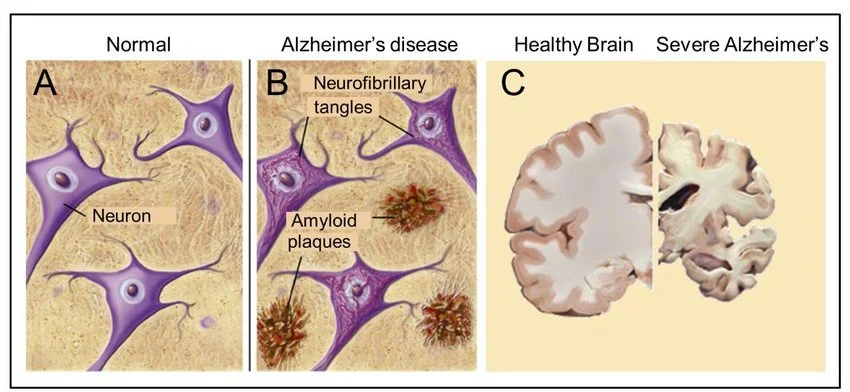
Normal neurons and brain tissue compared to those with amyloid plaque
What causes Alzheimer’s Disease and dementia?
While we do not know all of the exact mechanisms that create the disease, we are learning more about risk factors everyday. In a previous article, I have covered other risk factors for these diseases, including blood sugar dysregulation, bacterial pathogens and aluminum toxicity.
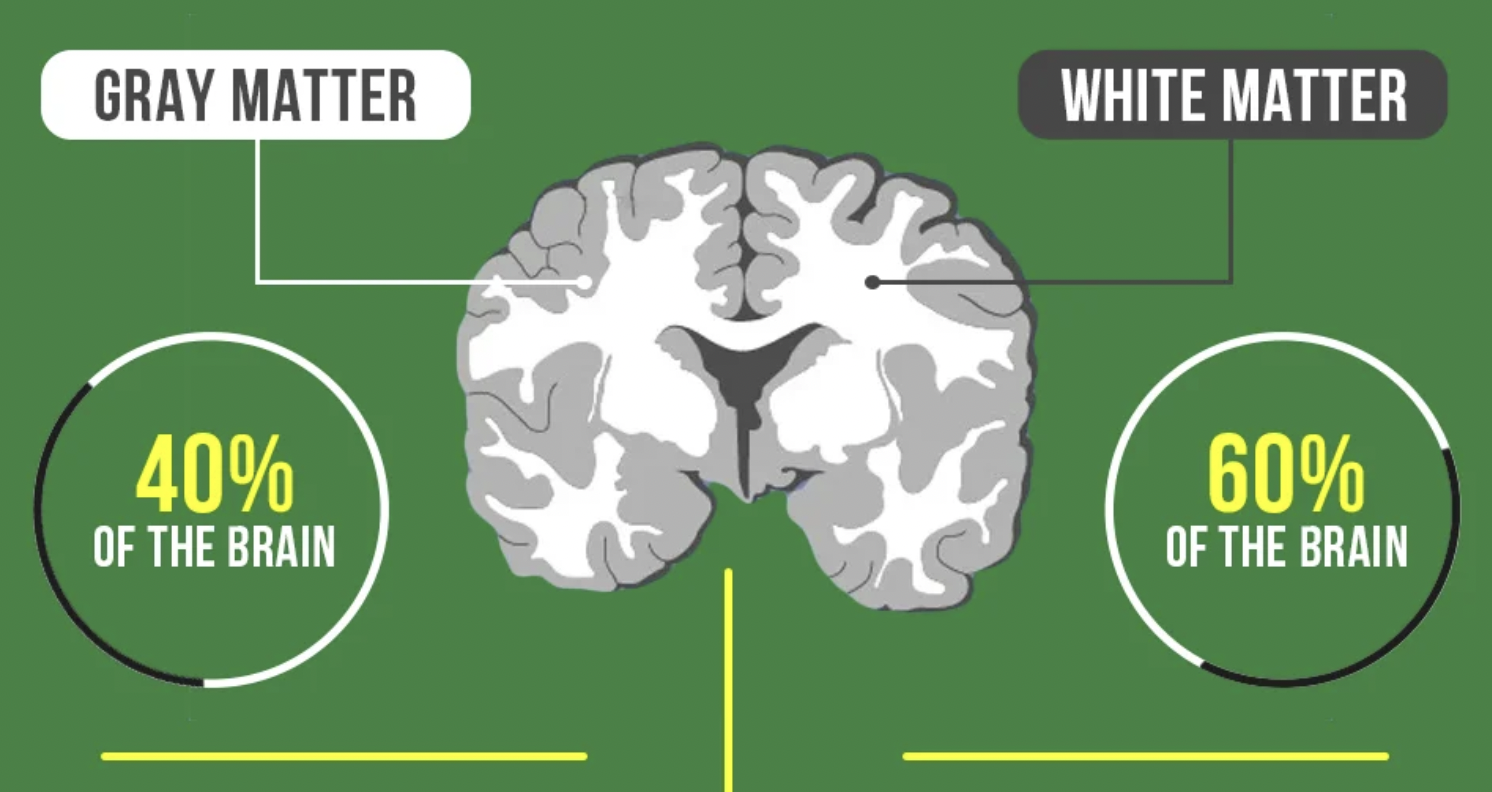
Ultimately, the great concern is the buildup of amyloid plaques, particularly amyloid Beta (Aß). The deposition of this plaque material creates death of neurons and glial cells in the brain, particularly in the gray matter which is responsible for cognition, memory and relaying information throughout the body.
Gray matter in the brain
Amyloid plaques have been referred to as “metal sinks” in the brain with high levels of zinc, copper and iron (ferritin-enriched). These metal ions aggregate (collect) in concentrated fashion outside of nerves and glial cells which require their presence for healthy functioning. A bit later, we will discuss what is required to alter this aggregation of metals, which also function as minerals in the body with critical chemical impacts.
Oxidative stress is at the heart of disease onset and progression - what is it?
Oxidative stress can occur when there is an imbalance of free radicals and antioxidants in the body. Antioxidants include vitamins A, C and E, as well as glutathione, astazanthin, lycopene and resveratrol.
It is normal for the body to produce free radicals during metabolic processes. However, cells also produce and/or utilize food-based antioxidants that neutralize these free radicals. In general, the body is able to maintain a balance between antioxidants and free radicals.
Several factors contribute to oxidative stress and excess free radical production. These factors include:
unhealthy diet and lifestyle
toxins and heavy metals
environmental factors such as pesticides, pollution and radiation
The body’s natural immune responses can also trigger oxidative stress temporarily. This type of oxidative stress causes mild inflammation that resolves after the immune system fights off an infection or repairs an injury. Occasionally, however, resolution does not occur. This is one of the reasons that I highlight pathogenic issues in my prior article on this topic - many people are unaware of chronic, low-grade pathogenic issues in their bodies that can prevent normalization of the immune response.
Uncontrolled oxidative stress can accelerate the aging process and may contribute to the development of a number of conditions, including the ones we are discussing here. Long-term oxidative stress damages the body’s cells, proteins, and DNA. In AD the deposition of Aβ is associated with oxidative stress.
Vitamin D and its role with dementia, AD and stroke risks
Brand new research is out this past week, reporting results from a large study of over 427,000 subjects. The results show a significant correlation between vitamin D deficiency and the risk for dementia. (For some basic background on vitamin D, please read here.)
Here are some of the body functions directly impacted by vitamin D:
blood sugar regulation
hormone health
serotonin and dopamine levels
reduction of virus survival and replication
reduction of inflammatory cytokines
reduction of respiratory distress/support of lung function and surfactants
support of adaptive and innate immune systems
T cell regulation
balance of calcium in gut and calcium & phosphorus in the blood and bones
support of neuromuscular function
As described in my vitamin D campaign linked above, the most effective and natural way to optimize vitamin D is through appropriate sun exposure. Vitamin D is also a hormone and is best synthesized through the stimulation of skin receptors via ultra violet (UV) light. This requires daily safe exposure at the height of the sun’s position overhead, without sunscreen (which blocks the skin reaction to UV light). When this type of practice is either impossible at certain times of the year or at certain latitudes, supplementation is a must, and the inclusion of vitamin K for absorption is a must (approximately 200 mcg’s daily). Please see the chart below for an excellent reference on supplementation and blood levels of vitamin D.
Adult levels of vitamin D and expected serum level
The study results indicate that vitamin D deficiency was associated with an increased risk of dementia and stroke, with the strongest associations for those with serum results of <25 nmol/L of 25-OH. This level is compared with levels of 50–75 nmol/L. In my practice, I recommend a level of 70-75.
Continuing the conversation about ionic metals - zinc, copper and iron
The 3 metal ions which have been studied in conjunction with dementia and AD are zinc, copper and iron. Let’s talk a bit about what these minerals do in the body.
Zinc is a trace mineral (meaning that the body only needs a small amount), and yet it is necessary for almost 100 enzymes to carry out vital chemical reactions. It is involved in the senses of taste and smell, and is a major player in the creation of DNA, growth of cells, building proteins, healing damaged tissue, aiding production of hydrochloric acid in the stomach, and supporting a healthy immune system. Because it helps cells to grow and multiply, adequate zinc is required during times of rapid growth such as childhood, adolescence, and pregnancy.
Copper is another essential trace mineral. (It is antagonized by zinc in both its nutritional and toxic forms.) It helps your body make red blood cells, protects nerve cells and helps to keep your immune system healthy. It is involved in collagen formation, a key part of bones and connective tissue. Copper may also act as an antioxidant, reducing free radicals that can damage cells and DNA. Copper helps the body absorb iron. Your body also needs copper to make energy. (Note that shellfish is an excellent source for both zinc and nutritional copper. Both zinc and copper are in high concentrations in the gray matter of the brain)
Lastly, iron is a major component of hemoglobin, a type of protein in red blood cells that carries oxygen from your lungs to all parts of the body. Without enough iron, there aren’t enough red blood cells to transport oxygen, which leads to fatigue. Iron is also part of myoglobin, a protein that carries and stores oxygen specifically in muscle tissues. Iron is important for healthy brain development and growth in children, and for the normal production and function of various cells and hormones.
Now, let’s take a look at the displacement of these metal ions/minerals in the face of amyloid plaque and dementia development.
Important research has shown that zinc and copper are found in high concentrations outside the brain cells, forming aggregate “pools” with Aβ. Copper and zinc are normally released into the neuronal synapse during neurotransmission, and are readily exchangeable. In a healthy state, these metals are taken back up into cells by specialized protein chaperones. The problem begins here - Aβ is also released into the synapse, potentially to modulate synaptic function. This makes the synapse a unique location where Aβ comes into contact with relatively high concentrations of “freely” available metals. This may explain why Aβ deposition and plaque formation is reported to begin in the synapse. Zinc shuttling appears to become fatigued in neurodegenerative diseases. Increased oxidative stress levels in Alzheimer’s Disease are also associated with the production of compounds which have the capacity to inhibit zinc export.
In particular with zinc, there are a number of transporters that carry it either into or out of the cytoplasm of the cells. In the brain, some of the crucial compounds that handle this trafficking of zinc carry it in tandem with glutamate, an excitatory neurotransmitter. It is likely that zinc is the counter-ion to glutamate in some nerve fibers and synapses, since it forms a complex that slowly dissociates upon release. This is akin to zinc loading of insulin in the secretory granules of pancreatic β-cells. - a mechanism that helps to regulate blood sugar.
Less is known and understood about copper metabolism in the brain, but what is clear is that the glutamatergic synapse is one of the only places in the body where freely exchangeable ionic copper appears. (Elemental copper exists in the cytoplasm of the body’s cells in its oxidation state of zero. It is extensively trafficked by a system of intracellular chaperones that prevent any free ionic copper from appearing in the cytoplasm, in order to prevent this reactive metal ion species from generating radicals and reactive oxygen.) Total cellular copper levels are decreased in the cortex of the brains of those with Alzheimer’s Disease. In contrast to these low levels, there is evidence that the remaining tissue copper is poorly joined, causing an elevation of labile, redox-active copper in the brain that is associated with the increased oxidative stress present in the brains of Alzheimer’s patients. The excess production of reactive oxygen species (ROS) created via Aβ’s reaction with copper is a key factor. The brain is a highly oxidative organ consuming 20% of the body's oxygen despite accounting for only 2% of the total body weight.
The concern with iron in our conversation is that excess iron creates high oxidative stress. Aβ binds heme and hemoglobin, which we mentioned earlier is important for the transport of iron and oxygen throughout the body. Hemoglobin accumulates in amyloid pathology in Alzheimer’s Disease. Aβ is detected at higher levels on red blood cells from AD patients compared to healthy controls. Iron export chemicals are also at lower levels in the disease. Indications are that disruptions in iron synthesis are present prior to the development of these diseases. As mentioned in my earlier article, pathogenic activity in the body and exhaustion/disruption of the spleen are important factors.
Research indicates that these same metal ions are influencing expressions of other types of neurodegenerative prion diseases such as Parkinson’s Disease.
Summations and Solutions
The authors of the study on metal ion aggregation also provided us with some hope for supporting better transport and intracellular levels of these important minerals. While their focus is on pharmaceutical drugs to accomplish this task, the ultimate function of those drugs can also be handled by specific natural compounds. The authors state: “we suspect that the timing of an elevation of iron, decrease in copper and fatigue of extracellular zinc clearance within the cerebral cortex in post-reproductive life may combine to create a change in metal milieu that induces proteostasis and neurotoxicity in these disorders”. This moves the diseases into the realm of metal metabolism disease and not just strictly proteinopathy disease, which has been the larger focus to date.
With regard to iron excess in the brain and plaque deposition, flavanoids such as curcumin have been shown to effectively chelate iron. Curcumin also effectively reduces reactive oxygen species (ROS) produced in oxidative stress.
To address zinc and copper aggregations, ionophores such as isoquercetin are utilized. Ionophores in this scenario can move metals trapped within extracellular Aβ aggregates and facilitate their uptake into the deficient intracellular space of neurons and glial cells where they are needed. Quercetin crosses the blood-brain-barrier, which few substances do effectively.
It should be noted that the aggregation and toxicity of Aβ are notoriously subject to variability, and factors such as pH, salinity and the presence of calcium and magnesium modulate its behavior in response to metal ions. This brings us to the all-important aspect of personalized nutrition in prevention and holistic healing of disease. You will find this category in all of my articles in summations and solutions, and the reason for that is that you are a unique biochemical individual. Your nutrient needs and metabolic processing are yours and yours alone. All of your homeostatic mechanisms (pH included) depend upon proper individualized nutrition. You may have higher needs for calcium or for magnesium, and your responses to foods and their pH effects will be unique to you as well. Metabolic Typing ® identifies your individual needs and helps you to meet your energy requirements for healing. It is the foundation of healing in which we optimize your production of ATP - the energy for all of your body’s cells.
Individualized testing with careful analysis is at the heart of our ability to prevent and/or modulate disease expression.
If you or someone you love is either at genetic risk for one of these neurodegenerative diseases, or is already expressing symptoms of disease, now is the time to investigate and bring effective solutions. Please contact me at Julie@truenaturehealthconsulting.com for more information and support. We provide holistic telehealth services.